Description
TB-500 (Thymosin Beta-4) Peptide
TB-500, or Thymosin Beta-4, is a synthetic analog of the endogenous Thymosin proteins, which are considered ubiquitously present in cells. The peptide belongs to a widespread family of 16 related molecules considered to exhibit a high degree of sequence conservation and localization in the majority of the tissues and circulating cells. TB-500 was developed with the intention of sequestering and blocking actin polymerization in eukaryotic cells.
Specifications
OTHER KNOWN TITLES: Thymosin Beta-4
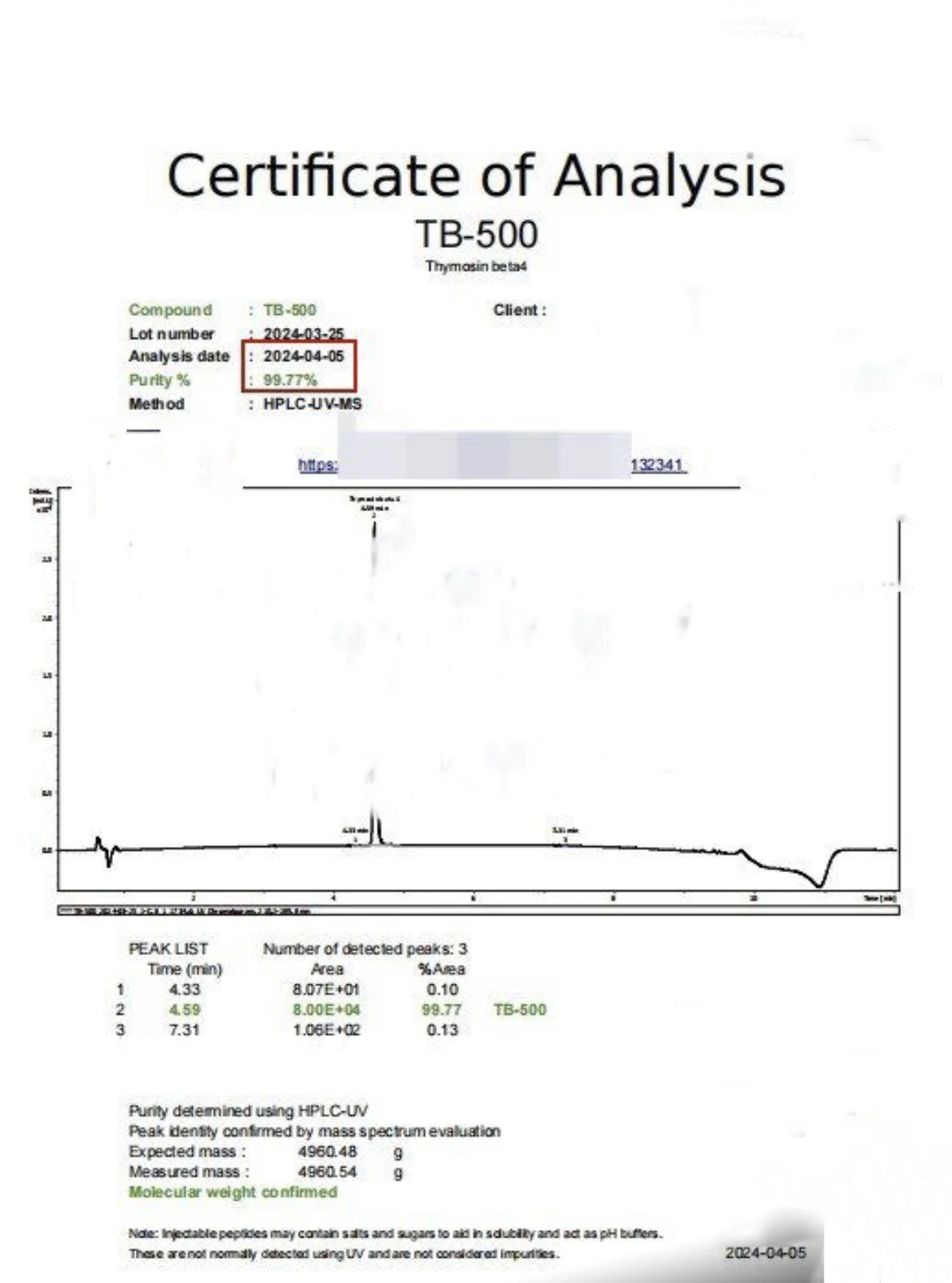
MOLECULAR FORMULA: C212H350N56O78S
MOLECULAR WEIGHT: 4963.506 g/mol
SEQUENCE: Ac-Ser-Asp-Lys-Pro-Asp-Met-Ala-Glu-lle-GluLys-Phe-Asp-Lys-Ser-Lys-Leu-Lys-LysThr-Glu-Thr-Gin-Glu-Lys-Asn-Pro-Leu-Pro-Ser-Lys-GluThy-lleGlu-Gin-Glu-Lys-Gin-Ala-Gly-Glu-Ser
TB-500 (Thymosin Beta-4) Research
TB-500, or Thymosin Beta-4 expression appears to increase four-six fold during early angiogenesis. Research suggests it may promote the growth of new blood cells from existing vessels, and thereby potentially mediate faster tissue repair.[1] Researchers point out that “Delineating the molecular pathways impacted by Tβ4 to promote vascular growth and remodeling may reveal novel targets for prevention … of vascular disease.” The actin-binding domain happens to be a short central stretch of amino acid. It appears to be involved in TB-500-mediated regulation of blood cell division, tissue repair processes, migration of endothelial cells and keratinocytes, and possibly also higher production of extracellular matrix-degrading enzymes.
TB-500 research suggests the peptide’s potential anti-inflammatory characteristics.[2] According to the research, “It acts by increasing angiogenesis and cell migration and is currently [studied in] wound repair.” TB-500, unlike other naturally produced growth factors, may possibly support endothelial and keratinocyte migration. It also does not appear to bind to the extracellular matrix, and researchers suggest that its low molecular weight may help it travel comparatively long distances through tissues. Study findings typically hypothesize the action of the peptide may be to regulate the polymerization and function of actin.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Dubé, K. N., & Smart, N. (2018). Thymosin β4 and the vasculature: multiple roles in development, repair and protection against disease. Expert opinion on biological therapy, 18(sup1), 131–139. doi:10.1080/14712598.2018.1459558
- Philp, D., Goldstein, A. L., & Kleinman, H. K. (2004). Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mechanisms of ageing and development, 125(2), 113–115. doi:10.1016/j.mad.2003.11.005
- Maar K, Hetenyi R, Maar S, et al. Utilizing Developmentally Essential Secreted Peptides Such as Thymosin Beta-4 to Remind the Adult Organs of Their Embryonic State-New Directions in Anti-Aging Regenerative Therapies. Cells. 2021;10(6):1343. Published 2021 May 28. doi:10.3390/cells10061343
- Malinda, K. M., Sidhu, G. S., Mani, H., Banaudha, K., Maheshwari, R. K., Goldstein, A. L., & Kleinman, H. K. (1999). Thymosin beta4 accelerates wound healing. The Journal of investigative dermatology, 113(3), 364–368. doi:10.1046/j.1523-1747.1999.00708.x
- Song, R., Choi, H. M., Yang, H. I., Yoo, M. C., Park, Y. B., & Kim, K. S. (2012). Association between serum thymosin β4 levels of rheumatoid arthritis patients and disease activity and response to therapy. Clinical rheumatology, 31(8), 1253–1258. doi:10.1007/s10067-012-2011-7


Reviews
There are no reviews yet.