Description
BPC-157
BPC-157 research on tissue repair processes, although study findings indicate that the compounds may use different biochemical pathways to achieve this potential. Researchers also hypothesize synergistic effects in the healing process.
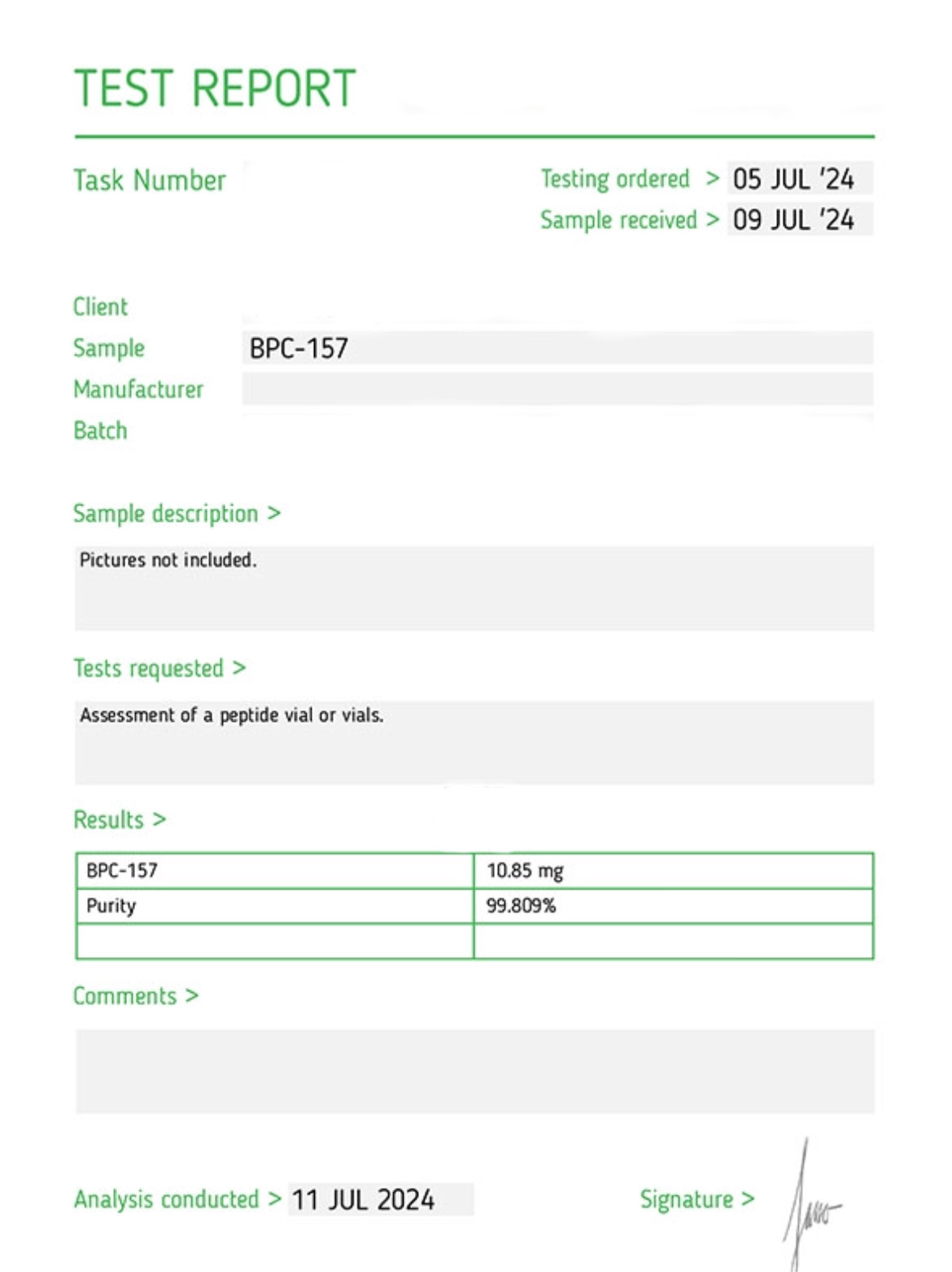
BPC-157 Specifications
OTHER KNOWN TITLES: BPC 157
MOLECULAR FORMULA: C62H98N16O22
MOLECULAR WEIGHT: 1419.5355 g/mol
SEQUENCE: L-Valine,glycyl-L-alpha-glutamyl-L-prolyl-L-prolyl-Lprolylglycyl-L-lysyl-L-prolyl-L-alanyl-L-alpha-aspartyl-L-alpha-aspartyl-L-alanylglycyl-L-leucyl-;glycyl-L-alpha-glutamyl-L-prolyl-L-prolyl-L-prolylglycyllysyl-L-prolyl-L-alanyl-L- alpha-aspartylL-alpha-aspartyl-L-alanylglycyl-L-leucyl-L-valine
BPC-157 & TB-500 Blend Research
BPC-157 :WOUND RESEARCH
BPC-157 is also known as Body Protection Compound-157. This pentadecapeptide comprises 15 amino acids and is similar in structure to a naturally occurring peptide of the same name, mainly found in gastric juice. BPC-157 has been studied for its potential in tissue repair. Laboratory studies on murine models have reported the healing of a transacted Achilles tendon in the presence of elevated levels of BPC-157. BPC-157 has also been suggested to stimulate blood flow, modulate nitric oxide levels, and support the healing of stomach ulcers and intestinal injuries. BPC-157 may exert potential action by upregulating growth factors such as early growth response gene-1 (EGR-1). The interaction between BPC-157 and early EGR-1 appears to involve specific molecular mechanisms.[6] In a study comparing the mechanism of action of BPC-157 with becaplermin (PDGF-BB), a peptide suggested for lower extremity ulcers, the expression of EGR-1 was investigated.
The study examined granulation tissue formation in sponge implantation in normoglycemic rats and healing of full-thickness excisional wounds in db/db genetically diabetic mice. Both PDGF-BB and BPC-157 were observed to exhibit similar selectivity for stimulating granulation tissue in both models. However, BPC-157 may have greater potential in promoting early collagen organization, as suggested by the research teams. Additionally, the researchers speculated that BPC-157 may have induced the expression of EGR-1 and its repressor, nerve growth factor 1-A binding protein-2 (nab2), in non-differentiated Caco-2 cells more rapidly than PDGF-BB. EGR-1 is a gene that is considered to respond immediately and is potentially involved in the generation of cytokines and growth factors, as well as the early formation of extracellular matrix components like collagen. The apparent stimulation of EGR-1 expression by BPC-157 may suggest a hypothetical mechanism by which it may possibly improve wound healing. There are also several proposed mechanisms regarding how TB-500 might potentially interact with wound healing processes. TB-500 introduction appears to influence cytokine production and accelerate wound healing in models of corneal wounds.[7] After injury, TB-500 seems to enhance the expression of IL-1β and IL-6 mRNA in murine model corneas. Additionally, TB-500 presentation following alkali injury may reduce the expression of chemoattractants for polymorphonuclear neutrophils (PMNs), MIP-2, and KC in mouse corneas, possibly resulting in reduced PMN infiltration. Regarding inflammatory signaling pathways in the cornea, TB-500 might mediate NFκB inflammatory signaling pathways, which could have anti-inflammatory effects. It has also been suggested that TB-500 has anti-apoptotic properties. Overexpression of TB-500 in cells appears to cause an increased growth rate, reduced basal apoptosis, and resistance to cell death-inducing factors. TB-500 may inhibit apoptosis in corneal epithelial cells by inhibiting caspases and suppressing the release of pro-apoptotic protein bcl-2 from mitochondria. The anti-apoptotic activity of TB-500 may involve the reduction of early cell death initiation signals and activation of the survival kinase Akt through complex formation with PINCH and integrin-linked kinase. TB-500 may exert an anti-apoptotic potential through multiple molecular pathways. However, it is important to note that these mechanisms are still speculative and require further research.
BPC-157: ANGIOGENIC ACTION
BPC-157 may exert angiogenic action. Several studies have attempted to shed light on the potential pro-angiogenic mechanism of BPC-157. BPC-157 appears to interact with the function of various growth factors, including vascular endothelial growth factor (VEGF). For example, one study using a chick chorioallantoic membrane (CAM) assay and an endothelial tube formation assay suggested that BPC-157 may have increased vessel density.[8] Moreover, the research team suggested that the peptide may have facilitated the recovery of blood flow in the ischemic muscle of the rat hind limb, as determined by laser Doppler scanning, indicating the compound’s potential to promote angiogenesis. Hypothetically, histological analysis of the hind limb muscle may have confirmed the increased number of vessels and possibly enhanced vascular expression of vascular endothelial growth factor receptor 2 (VEGFR2) with BPC-157 influence. Further investigations using vascular endothelial cells suggested that BPC-157 might have increased mRNA and protein expressions of VEGFR2, but not VEGF-A. Additionally, BPC-157 possibly promoted the internalization of VEGFR2 in vascular endothelial cells. Furthermore, BPC-157 may have activated the VEGFR2-Akt-eNOS signaling pathway time-dependently. Therefore, it is posited that BPC-157 may exhibit pro-angiogenic action by potentially promoting the expression and internalization of VEGFR2 and potentially activating the VEGFR2-Akt-eNOS signaling pathway. The researchers concluded that “BPC 157 promotes VEGFR2 internalization in association with VEGFR2-Akt-eNOS activation.”
BPC-157: GROWTH HORMONES, SYNERGISM
Growth hormone may be a critical mediator in BPC-157 when observed in the context of wound healing, as both molecules appear to interact with the growth hormone during the healing process. BPC-157 has been suggested by researchers to induce a higher production of growth hormone receptors on fibroblasts’ surfaces, possibly increasing the lifespan of these cells.[10] This process, in turn, might promote the regeneration of soft tissues. It appears to ensure that the actin reserve is well maintained for use by fibroblasts in their extended lifespans. Thus, BPC-157, collagen, and growth hormones may potentially form an effective synergistic approach in tissue repair research.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Lee, E., & Padgett, B. (2021). Intra-Articular Injection of BPC 157 for Multiple Types of Knee Pain. Alternative therapies in health and medicine, 27(4), 8–13.
- Chang, C. H., Tsai, W. C., Lin, M. S., Hsu, Y. H., & Pang, J. H. (2011). The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. Journal of applied physiology (Bethesda, Md. : 1985), 110(3), 774–780. https://doi.org/10.1152/japplphysiol.00945.2010
- Moon, E. Y., Im, Y. S., Ryu, Y. K., & Kang, J. H. (2010). Actin-sequestering protein, thymosin beta-4, is a novel hypoxia responsive regulator. Clinical & experimental metastasis, 27(8), 601–609. https://doi.org/10.1007/s10585-010-9350-z
- Zhou, B., Honor, L. B., Ma, Q., Oh, J. H., Lin, R. Z., Melero-Martin, J. M., von Gise, A., Zhou, P., Hu, T., He, L., Wu, K. H., Zhang, H., Zhang, Y., & Pu, W. T. (2012). Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. Journal of molecular and cellular cardiology, 52(1), 43–47. https://doi.org/10.1016/j.yjmcc.2011.08.020
- Tokura, Y., Nakayama, Y., Fukada, S., Nara, N., Yamamoto, H., Matsuda, R., & Hara, T. (2011). Muscle injury-induced thymosin β4 acts as a chemoattractant for myoblasts. Journal of biochemistry, 149(1), 43–48. https://doi.org/10.1093/jb/mvq115
- Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007 Sep 10;570(1-3):212-21. doi: 10.1016/j.ejphar.2007.05.072. Epub 2007 Jun 16. PMID: 17628536.
- Sosne G, Qiu P, Kurpakus-Wheater M. Thymosin beta 4: A novel corneal wound healing and anti-inflammatory agent. Clin Ophthalmol. 2007 Sep;1(3):201-7. PMID: 19668473; PMCID: PMC2701135.
- Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JS. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl). 2017 Mar;95(3):323-333. doi: 10.1007/s00109-016-1488-y. Epub 2016 Nov 15. PMID: 27847966.
- Lv S, Cai H, Xu Y, Dai J, Rong X, Zheng L. Thymosin‑β 4 induces angiogenesis in critical limb ischemia mice via regulating Notch/NF‑κB pathway. Int J Mol Med. 2020 Oct;46(4):1347-1358. doi: 10.3892/ijmm.2020.4701. Epub 2020 Aug 11. PMID: 32945357; PMCID: PMC7447324.
- Chang, C. H., Tsai, W. C., Hsu, Y. H., & Pang, J. H. (2014). Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules (Basel, Switzerland), 19(11), 19066–19077. https://doi.org/10.3390/molecules191119066

Reviews
There are no reviews yet.